Electrical Charge and Material Handling
Powders and granular materials can acquire electrical charge on the surface of their particles due to contact and movement against handling equipment and containers. They can also acquire charge due to contact and movement of particles within the material itself. This process is called tribocharging. Tribocharging is caused by electrons moving from one surface to another when different materials come in contact with each other. One material will become positive and the other will become negative. The amount of charge developed depends on the nature of the materials in contact, the pressure of the contact, the relative velocity of the contact surfaces, and the friction between the contact surfaces.
Measuring the charge acquisition properties of powders and granular materials is important because charge acquisition leads to problems and unstable behavior. Charged materials stick to processing equipment and containers. Charged materials can become airborne more easily. Charge materials flow in different ways than materials with no charge. In fact, many research believe that material electrical properties are the most important contributors to powder flow behavior.
|
Charge can cause powder particles to stick to one another and to equipment surfaces creating blockages and cleaning problems |
Charge can cause particles to repel one another creating airborne dust and materials that are difficult to control |
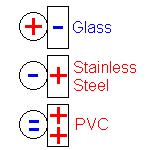
Mercury Scientific Inc offers the ION Charge Module to measure the charge acquisition properties of powders and granular materials. The ION module can be added to the Volution Powder Flow Tester or The Revolution Powder Analyzer. Both options allow test samples of powder and granular materials to be charged with contact surfaces of various materials including stainless steel, glass, aluminum, or plastic. Custom materials are also available and users can create their own contact surfaces like paper or coated materials.
Understanding charge acquisition properties can lead to corrective action to eliminate charge problems and unpredictable powder behavior. Corrective action can include changing the moisture content of a material, changing the particle size or particle size distribution of a material, adding a flow agent or glidant to a material to mitigate charge development, or changing the surfaces that the materials come in contact with.
|
Changing the particle size of particles in a powder can change their charge acquisition properties |
Surface moisture in a powder or granular material can dissipate charge |
|
Flow agents and glidants can dissipate charge in a powder or prevent charge from accumulating |
Contact materials can create or remove charge from powders and granular materials. |
Using the ION Charge Module with the Revolution allows measurement of charge acquisition properties between contact surfaces and test samples while controlling velocity and contact time.
|
Step 1: Test sample is loaded into the sample drum and placed in the analyzer on the two rollers. |
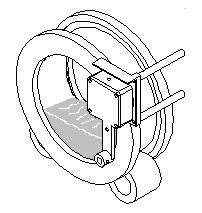 |
|
Step 2: The field meter is rotated in front of the sample drum and the initial charge on the surface of the contact plate is measured. |
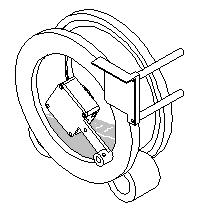 |
|
Step 3: The sample drum is rotated at a programmed velocity and the charge on the contact plate is measured at programmable intervals. |
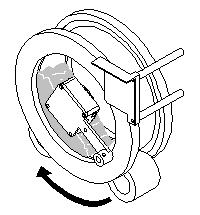 |
|
Step 4: The drum rotation is stopped and the field meter measures the charge dissipation. |
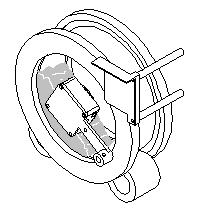 |
Experimental Data
Charge Versus Particle Size
-
Sample
Charge
D50 = 4um
3708 V
D50 = 8.2um
3009 V
D50 = 11-15um
2303 V
D50 = 16um
1516 V
The data above is for a powder with different particle sizes charged with glass. Typically charge development increases as particle size decreases.
Charge Versus Moisture Content
-
Sample
Charge
0.7% Moisture
2006 V
0.9% Moisture
1098 V
1.2% Moisture
731 V
2.9% Moisture
43 V
The data above is for a powder with different moisture content charged with glass. Typically charge development decreases as moisture content increases.
Charge Versus Flow Aid Concentration
-
Sample
Charge
No Flow Aid
-1260 V
0.4% Flow Aid
240 V
0.8% Flow Aid
1310 V
The data above is for a powder with different flow aid concentrations charged with polycarbonate. Typically charge development changes as flow aid content changes.
Charge Versus Surface Treatment
-
Sample
Charge
No Surface Treatment
-1367 V
0.05% Surface Treatment
1257 V
0.15% Surface Treatment
2007 V
The data above is for a powder with different concentrations of surface treatment liquid charged with glass.